Abstract
Burkitt's lymphoma is an incredibly aggressive B-cell derived non-Hodgkin lymphoma that is quickly fatal if left untreated. Doxorubicin (Doxo) provides one method of treatment for this cancer, but has numerous common side effects including significant weight loss, nausea, hair loss, bone marrow suppression, vomiting, rash, heart damage, allergic reaction, tissue damage at injection site, and treatment-related leukemia. Therefore, better therapies which deliver Doxo specifically to tumor cells hold great promise for a more effective therapy with reduced side-effect severity. Carbon nanotubes (CNTs) have long been of interest as drug delivery transporters because of their unique structure, high drug-binding surface area, and variety of customization options. Unfortunately, most CNTs have proven toxic during in vivo studies because of the presence of high residual contaminants and near-unavoidable aggregative properties. A novel form of clean, discrete CNT (dCNT) marketed as Medical Grade MOLECULAR REBAR® (MGMR®) lack the aggregative problems and high toxin levels of CNT before them and show promise as a drug delivery technology.
We observed no toxicity of dCNTs in vitro across multiple cell types or in vivo in mice establishing a high MTD of at least 70 mg/kg by an i.v. route. Biodistribution studies of Lu-177 radioisotope labeled dCNTs (single tail vein injection into BALB/C female mice) demonstrated tolerability and clearance from various organs. Mice were sacrificed at 7 timepoints under 34d and many organs were analyzed for accumulation. The liver, spleen, and blood identified one route of clearance and distribution, but it was interesting to note that the bone retained ~10% of injected dCNTs at all time points, demonstrating potential for bone targeted therapies. Further targeting to the bone was possible by grafting commonly available bone chelators to the surface of the dCNT to create BT-dCNT as shown by significantly great adhesion (p<0.05) to ex vivo bone.
Protocols were then developed to saturate the surface of the dCNT with therapeutic Active Pharmaceutical Ingredients (API) and then characterize their elution/release over time. We then optimized methods for drug-loading and observed a slow drug release profile for most drugs. Enhanced pharmacokinetic information was tested in cell culture and then confirmed by animal experimentation. In addition, cell penetration and delivery study of MGMR® in vitro showed that endocytosis is nontoxic and well tolerated and that small peptides can be delivered to cancer cells and retain their functionality. Zeta Potential measurements (-40 to -50 mV) and Dynamic Light Scattering validated the stability of different dCNTs over time.
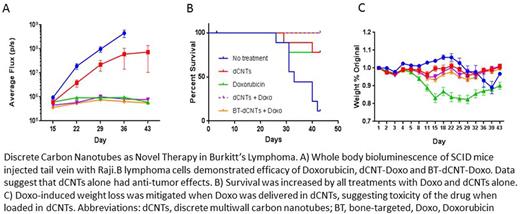
Lastly, in a Raji-Burkitt's lymphoma mouse model, we saw significant increases in survival, and decreases in tumor burden in mice treated with Doxo (free, in dCNTs, and in BT-dCNTs vs no drug control). SCID mice were inoculated via the tail vein with 2x105 Raji B. luc+ tumor cells and treated once a week with one of the following: Doxo (5 mg/kg), dCNTs (14.29 mg/kg), dCNTs (14.29 mg/kg) + Doxo (5 mg/kg), BT-dCNTs (14.29 mg/kg) + Doxo (5 mg/kg), or no treatment. Using whole body bioluminescence imaging (BLI), we saw that the groups receiving both the Doxo-loaded dCNTs and the Doxo-loaded BT-dCNTs had greatly reduced tumor burden over time compared to untreated groups, and a tumor burden similar to those that received free Doxo alone (Figure 1A). Both groups receiving Doxo-loaded dCNTs also showed increased survival compared to the Doxo alone group (Figure 1B). Importantly, the typical signs of Doxo toxicity in mice, characterized by rapid weight loss or drug-related mortality, were not seen in the groups that received Doxo-loaded CNTs (Figure 1C).
In summary, dCNTs is a highly promising biomedical tool for drug delivery for clinical applications. Our dCNTs represent a promising platform for use in biomedical research as they have proven to be nontoxic, tunable, stable, and capable of drug delivery and organ-specific targeting. This novel technology gives great promise for the bone-targeting ability of novel carbon nanotubes in vivo as a bone-specific drug delivery mechanism for diseases that grow in bone or bone marrow specifically.
Falank: BioPact Ventures, LLC: Research Funding. Tassett: BioPact Ventures, LLC: Employment. Harris: BioPact Ventures, LLC: Research Funding. Farrell: BioPact Ventures, LLC: Research Funding. Everill: BioPact Ventures, LLC: Employment. Reagan: BioPact Ventures, LLC: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal